Molar Mass Of An Acid

Molar mass of H2SO4 (Sulfuric acid) is 98.072 g/mol .
Well, at present yous accept come to know the molar mass of H2SO4.
But how can yous become this value?
Let me bear witness you lot the calculation to become the molar mass of H2SO4 (Sulfuric acid).
H2SO4 (Sulfuric acid) Molar Mass Calculation
If y'all have a periodic table with you lot, then you tin easily calculate the tooth mass of H2SO4 (Sulfuric acid).
Because the molar mass of any molecule (or chemical compound) tin be calculated by simply adding the molar masses of individual atoms.
At present here we have to find the molar mass of H2SO4 (Sulfuric acid).
So for that, accept a look at the periodic table given below.
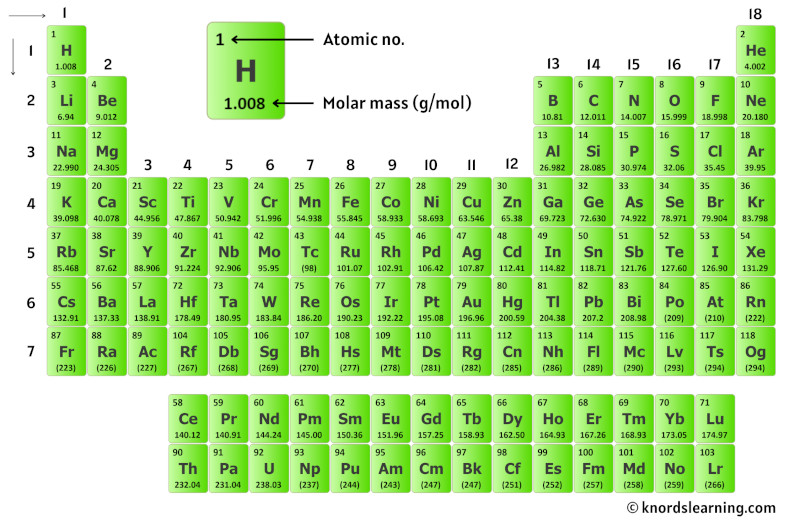
You tin see the tooth mass value of all the atoms from this periodic table.
Now in H2SO4, there are 2 Hydrogen atoms, 1 Sulfur atom and 4 Oxygen atoms.
So let'south wait at the molar mass of Hydrogen, Sulfur and Oxygen from the above periodic tabular array.
You tin meet that;
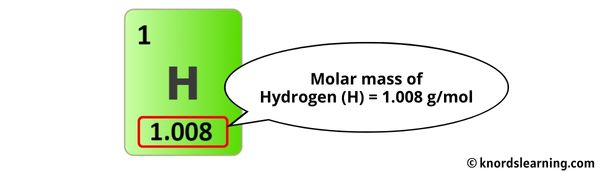
The molar mass of Hydrogen is 1.008 g/mol.
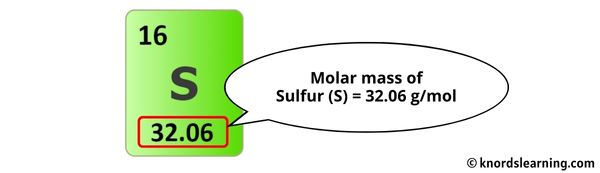
The molar mass of Sulfur is 32.06 thousand/mol.
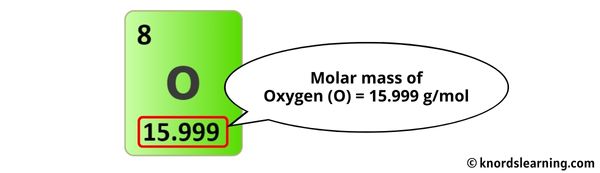
The molar mass of Oxygen is 15.999 one thousand/mol.
At present, to calculate the molar mass of H2SO4, y'all merely have to add the molar mass of all the individual atoms that are present in H2SO4.
You can run across that in H2SO4, there are two Hydrogen atoms, 1 Sulfur atom and 4 Oxygen atoms.
So, Molar mass of H2SO4 = Molar mass of 2 Hydrogen (H) atoms + Tooth mass of one Sulfur (S) atom + Tooth mass of 4 Oxygen (O) atoms.
= (ane.008) 2 + 32.06 + (15.999) 4
= 2.016 + 32.06 + 63.996
= 98.072 g/mol
Hence the Molar mass of H2SO4 is 98.072 k/mol .
I hope you have understood the brusque and uncomplicated calculation for finding the molar mass of H2SO4.
Remember
- In some books, yous may see the unit of molar mass as grams/mole or 1000/mole. Merely all these units (i.e g/mol, grams/mole and g/mole) are the same.
- Always follow the calculation club to avoid any mistakes in calculation. First solve the brackets, and then multiplications and at last practise the terminal improver.
- And don't forget to put the unit g/mol to your final calculated tooth mass.
Bank check out other related topics for more than do;
Glucose (C6H12O6) Tooth Mass
KCl (Potassium chloride) Molar Mass
Acetic acid (CH3COOH) Tooth Mass
Sodium carbonate (Na2CO3) Molar Mass
NaHCO3 (Sodium bicarbonate) Molar Mass
Molar Mass Of An Acid,
Source: https://knordslearning.com/h2so4-sulfuric-acid-molar-mass/
Posted by: coatestherds.blogspot.com


0 Response to "Molar Mass Of An Acid"
Post a Comment